A chemical equation which does not follow the law of conservation of mass is called skeleton equation. The reaction produces moles of barium chloride and moles of water.

Ba Oh 2 Hcl Bacl2 H2o Balanced Chemical Equation
So the correct option is B.
. 2HCl aq Ba OH2 aq BaCl2 aq 2H2 0 1 If 4 moles of barium hydroxide react The reaction consumes moles of. Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide. In a balanced equation the number of atoms of each element will remain equal.
Barium Hydroxide molecular weight. The balanced equation for this reaction is. The balanced equation for this reaction is.
So the law of conservation of mass is the principle behind balancing a chemical equation. Barium chloride Sulphuric acid Barium Sulphate Hydrochloric acid. Ba OH 2 2HCl BaCl 2 2H 2 O.
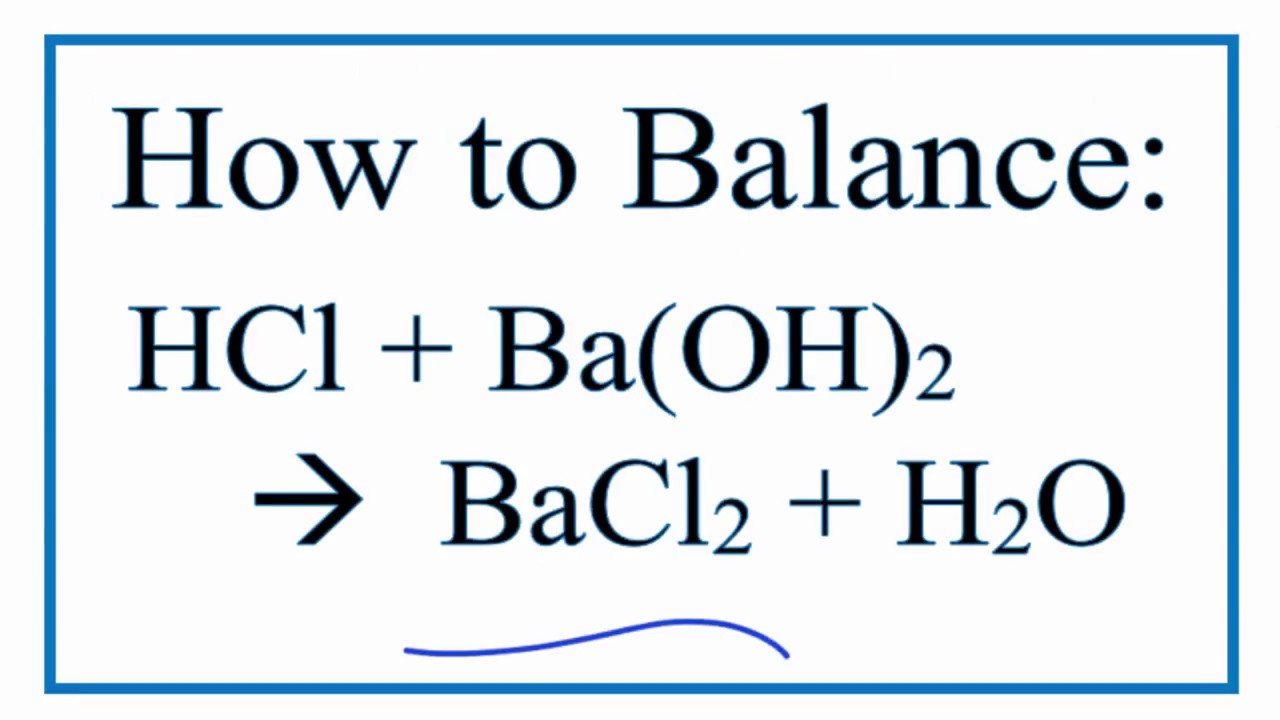
2HI Ba OH2 -- 2H2O BaI2. Barium hydroxide reacts with hydrochloric acid to form barium chloride and water. 2HCl aq Ba OH2 aq BaCl2 aq 2H2 0 1 If 4 moles of barium hydroxide react The reaction consumes moles of hydrochloric acid.
The balanced molecular equation is written below Explanation. The regular reaction equation is. 2HClaq BaOH2aq BaCl aq 2H O1 If 6 moles of hydrochloric acid react The reaction consumes moles of barium hydroxide.
Hydrogen chloride - diluted solution. The full ionic equation is. B a 2 H C l B a C l 2 H 2 When barium reacts with hydrochloric acid it will form barium chloride with the evolution of hydrogen gas.
Barium hydroxide hydrochloric acid balanced equation. The balanced equation for this reaction is. Ba OH2 aq 2HCl aq -- BaCl2 aq 2H2O l Wiki User.
HClBa OH2BaCl2H2O Balanced EquationHydrochloric acid aq barium hydroxide aq Balanced Eq. 2Haq 2CN-aq Ba2aq 2OH-aq -- BaCN2s H2O. Ba 2HCl BaC l 2 H 2.
2 HCl aq BaOH2 aq BaCl2 aq 2 H20 1 We can interpret this to mean. Check the balance Barium hydroxide react with hydrogen chloride to produce barium chloride and water. 2 moles of hydrochloric acid and moles of barium hydroxide React to produce.
Barium hydroxide hydrochloric acid balanced equation. A molecular equation is the chemical equation in which the ionic compounds are written as molecules rather than component ions. Here 1 mole of barium reacts with 2 moles of hydrochloric acid to produce 1 mole of barium chloride and 1 mole of hydrogen gas.
The balanced chemical equation for the reaction between hydrochloric acid and barium hydroxide is. Neutralization reaction is defined as the chemical reaction when an acid reacts with a base to produce a salt and water molecule. It decomposes to barium oxide when heated in a vacuum.
2HCN BaOH2 -- BaCN2 2H2O. See answer 1 Best Answer. Write the balanced chemical equations of the following word equation.
Moles of barium chloride and moles of water. When hydrochloric acid reacts with barium hydroxide barium chloride and water are produced. Use the Reference TO When nitrogen monoxide reacts with hydrogen nitrogen and water are produced.
Write a balanced equation for the reaction below.

Solved When Hydrochloric Acid Reacts With Barium Hydroxide Chegg Com

Hcl Ba Oh 2 Bacl2 H2o Balanced Equation Hydrochloric Acid Aq Barium Hydroxide Aq Balanced Eq Youtube

How To Balance Hcl Ba Oh 2 Bacl2 H2o Hydrochloric Acid Plus Barium Hydroxide Youtube
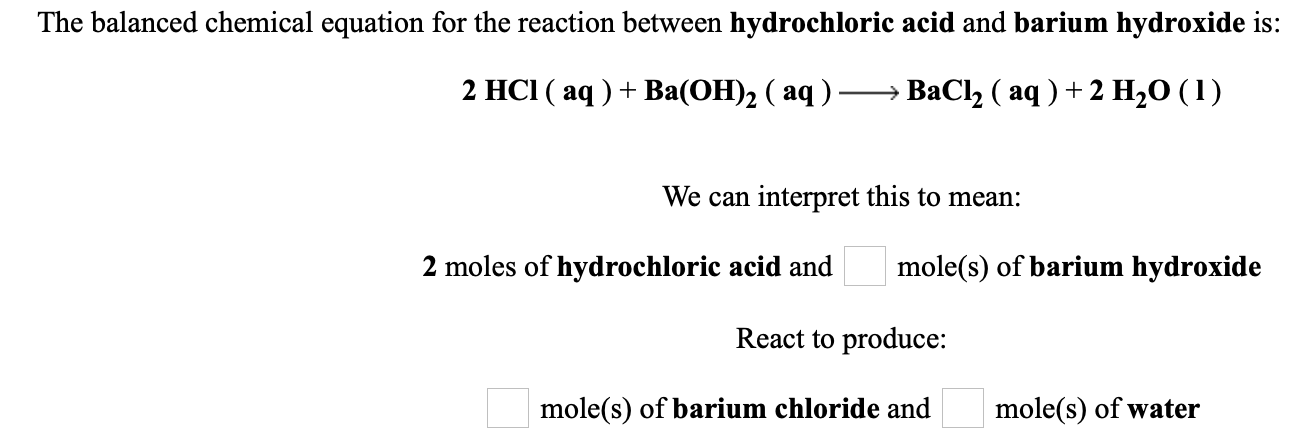
Solved The Balanced Chemical Equation For The Reaction Chegg Com
0 Comments